LMC focuses its research around three main topics :
- CARBON isotope LABELING
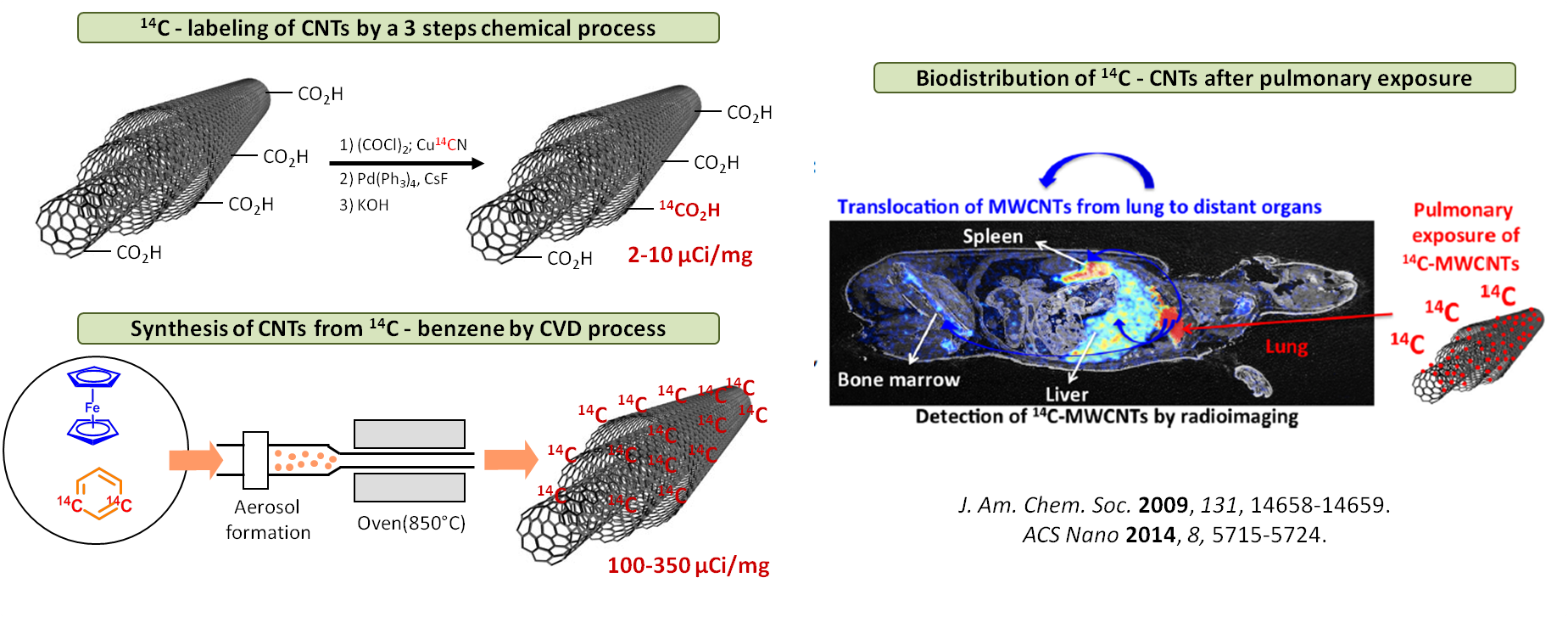
Carbon-14 isotopic labeling is a longstanding goal of our research group. Carbon-14 radiolabeling is a common procedure frequently used to understand the distribution, metabolism, toxicity of a drug in vivo, in pre-clinical and early clinical studies (Phase 0/1). As part of FDA’s safety assessment process, more than 80% of all drugs have used radioactive materials in their testing program.
Our efforts in this field are focused on the development of new multi-step chemical procedures able to increase the labelling efficiency of biologically active molecule, as well as to reduce the production of radioactive waste.
We are also actively involved in the development of new methodologies for 14C-labeling of nanoparticules and nanotubes in order to study their metabolism, in vivo biodistribution and toxicity.
- Bio-orthogonal Chemistry
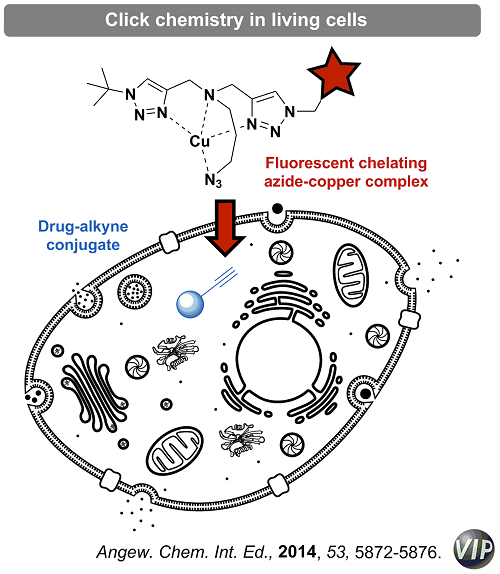
The development of chemical reactions that can be performed in living systems (i.e. cells, model organisms) has long held unique fascination in the field of chemical biology.
A bio-orthogonal reaction is characterized by the reaction of two functionalities, which will react under mild physiological conditions and are inert towards the biological environment. Quintessential example is the copper-catalyzed azide-alkyne cycloadditions (CuAAC).
We have recently applied the concept of chelation-assisted copper catalysis to the development of new azides that display unprecedented reactivity in the CuAAC, reacting almost instantaneously with alkynes under diluted conditions and in complex biological media.
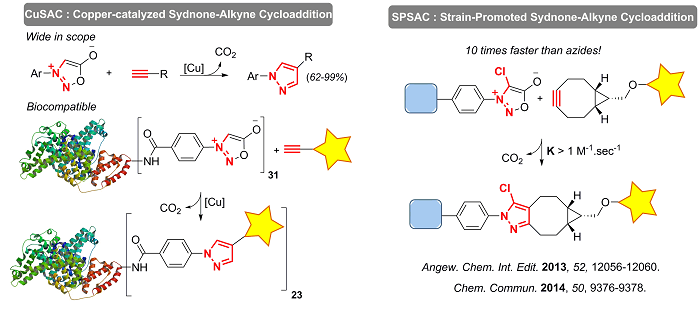
Driven by our in-house sandwich immunoassays high-throughput screening, we identified two new click reactions: the Copper-catalyzed Sydnone-Alkyne Cycloaddition (CuSAC) and the Strain-Promoted Sydnone-Alkyne Cycloaddition (SPSAC).
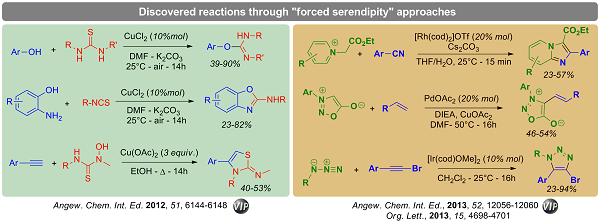
- HIGH THROUGHPUT REACTION DEVELOPMENT
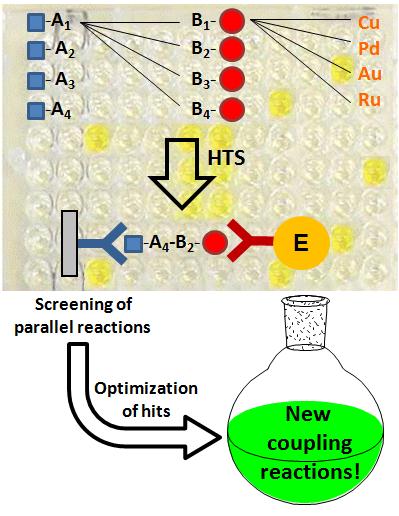
The discovery of new chemical reactions is a long-standing
goal of organic chemists. Recently, forced serendipity approaches have emerged as valuable alternative to purely rational approaches.
Over the last decade, we developed an unprecedented sandwich immunoassay screening which enables the high-throughput discovery and optimization of catalytic reactions.
This method, which allows the screening of more than 1000 catalysts or reaction conditions in a single day, has been successfully applied to new reaction discovery as well as asymmetric catalysis.