The identification of biomarkers is essential for
understanding the biological processes involved in neurodegenerative diseases.
Historically, the atrophy of certain brain structures has been proposed as a
biomarker of interest. However, despite its robustness and ease of measure,
cerebral structure atrophy appears in reality to provide little
pathophysiological information; it instead most likely reflects the long-term
effects of long-past biological events.
In a study published in NMR in Biomedicine, the
Magnetic
Resonance Methods to Study the Brain In Vivo team from the Neurodegenerative
Diseases Laboratory of MIRCen
(CEA-Jacob) identified pertinent early biomarkers of neurodegeneration,
particularly in the setting of Huntington's disease*. The team's researchers
developed a high magnetic field (11.7 Tesla) acquisition protocol combining the
biological specificity offered by proton magnetic resonance spectroscopy and
the high spatial resolution offered by gluCEST imaging (metabolic imaging of
glutamate).
They studied two murine models of Huntington's
disease, each presenting specific characteristics. The first was the Ki140CAG
model, characterized by slow disease progression similar to presymptomatic
disease forms observed in humans, and the second the R6/1 model, which reflects
juvenile, aggressive disease.
By combining the spectroscopy and metabolic imaging
techniques, the team was able to demonstrate striking differences between those
two models. In the R6/1 aggressive disease model, the quantity of the
metabolite N‐acetyl‐aspartate, found mainly in neurons, was shown to be
diminished, suggesting an alteration of the neuronal compartment. Furthermore,
the reduction in N‐acetyl‐aspartate was correlated with atrophy of the
striatum, a structure affected early in Huntington's disease. In contrast, the
quantities of other metabolites remained stable, suggesting that astrocytes
(another type of brain cell) were not affected and that energy metabolism was
preserved. In the Ki140CAG progressive disease model, modifications in the
rates of several metabolites may have reflected changes that were occurring
gradually in the mice's brains to compensate for deficits that developed
earlier in their lives. The GluCEST imaging studies provided measures that
would have been inaccessible to NMR spectroscopy. For example, the former detected
alterations in unexpected regions of the brain, such as the corpus callosum.
The MIRCen team showed
that the combinatorial complementarity of the spectroscopy and metabolic
imaging techniques can provide different angles of view on the course of Huntington's
disease. Their results may contribute to improving the use of animal models to
study a large spectrum of neurodegenerative diseases and evaluate the efficacy
of future treatments.
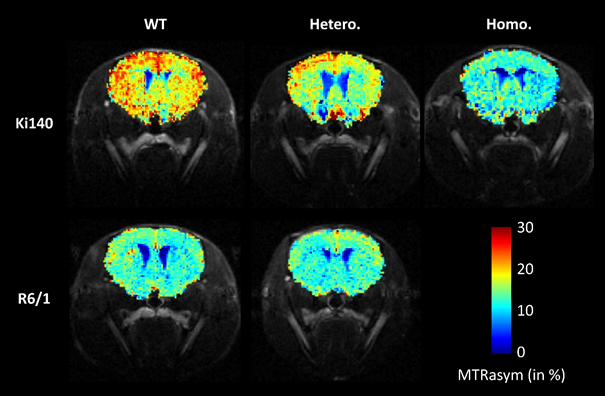
Metabolic imaging of glutamate (gluCEST) in Ki140CAG
(top row) and R6/1 (bottom row) mice. Ki140CAG mice homozygous for the muted
huntingtin gene (Homo., right column) show lower glutamate levels than do wild
type mice (WT, left column). Heterozygous mice (Hetero., middle column) show
intermediate levels, suggesting a correlation between glutamate levels and
disease severity. In contrast, the glutamate levels in heterozygous R6/1 mice
are comparable to those of wild type mice. This observation appears to be
peculiar to the R6/1 model and was brought to light by the protocol developed
for the study. © J. Flament / MIRCen
* Huntington's disease is a rare hereditary
disease for which there is currently no curative treatment. Patients with it
experience neuronal degeneration in areas of the brain involved in motor,
cognitive and/or behavioral functions.