Myeloproliferative neoplasms (MPNs) are a family of rare chronic blood diseases that evolve throughout the life of the patient and cause serious vascular and hematological complications. A major characteristic of these diseases is that they all show mutations in several genes, including JAK2, MPL and CALR. MPNs occur on a background of complex interactions that cause an initial phase of clonal expansion in the myeloid lineage¹, an inflammatory syndrome and bone marrow ("myelo") fibrosis. The "classic" MPNs are chronic myelogenous leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF).
Myelofibrosis (MF), be it primary or secondary², is characterized by the failure of blood cell production in the bone marrow, which is progressively invaded by reticulin and collagen fibers. Clinically, MF presents as an alteration of the general condition of the patient, with weight loss, cytopenia, anemia and spleen enlargement.
Severe MF has a poor prognosis, with increased disease-associated morbidity comprising such complications as thrombosis, hemorrhaging, infections and progression to acute leukemia. Survival in severe MF is about six years. Current treatment is mainly palliative and intended to lessen symptoms. The only available potentially curative treatment is an allogeneic hematopoietic stem-cell transplant.
With the goal of finding new therapeutic possibilities in this setting, researchers from IDMIT's Stem Cells and Therapeutic Applications Laboratory (LCSAT) took inspiration from their preceding work on therapies for CML³ and focused their attention on the PPAR-γ/STAT-5 pathway in MF.
In a collaborative study⁴ published in the Journal of Clinical Investigation, they evaluated the potential ability of agonists (ligands) for the nuclear receptor PPAR-γ to influence the development of MF. The agonists they tested already had market authorizations (MA): pioglitazone, a medication used in type 2 diabetes; and mesalazine, used in gut diseases involving chronic inflammation.
Tests were done both in vitro on hematopoietic cell lines and ex vivo on primary cells harvested from patients with MF. These tests shed light on the mechanisms underlying the antiproliferative action of PPAR-γ on hematopoietic progenitor cells. The team then performed additional in vivo analyses on the proliferation of myeloid lineages, employing three preclinical murine models of myelofibrosis: the human PV model JAK2V617F, the EV/MF model CALRdel52 and the myeloproliferative disorder model TPOhigh.
In the mouse models, treatment with PPAR-γ agonists prevented the decrease in hemoglobin⁵ levels associated with the development of the pathology and limited remodeling of the bone marrow. Finally, the LCSAT team showed that PPAR-γ reduces myeloproliferatation⁶ (notably via reduced STAT5 activity), modulates proinflammatory cytokines and protects the bone marrow stroma⁷ by blocking the activity of TGF-β, a major cytokine in fibrosis development.
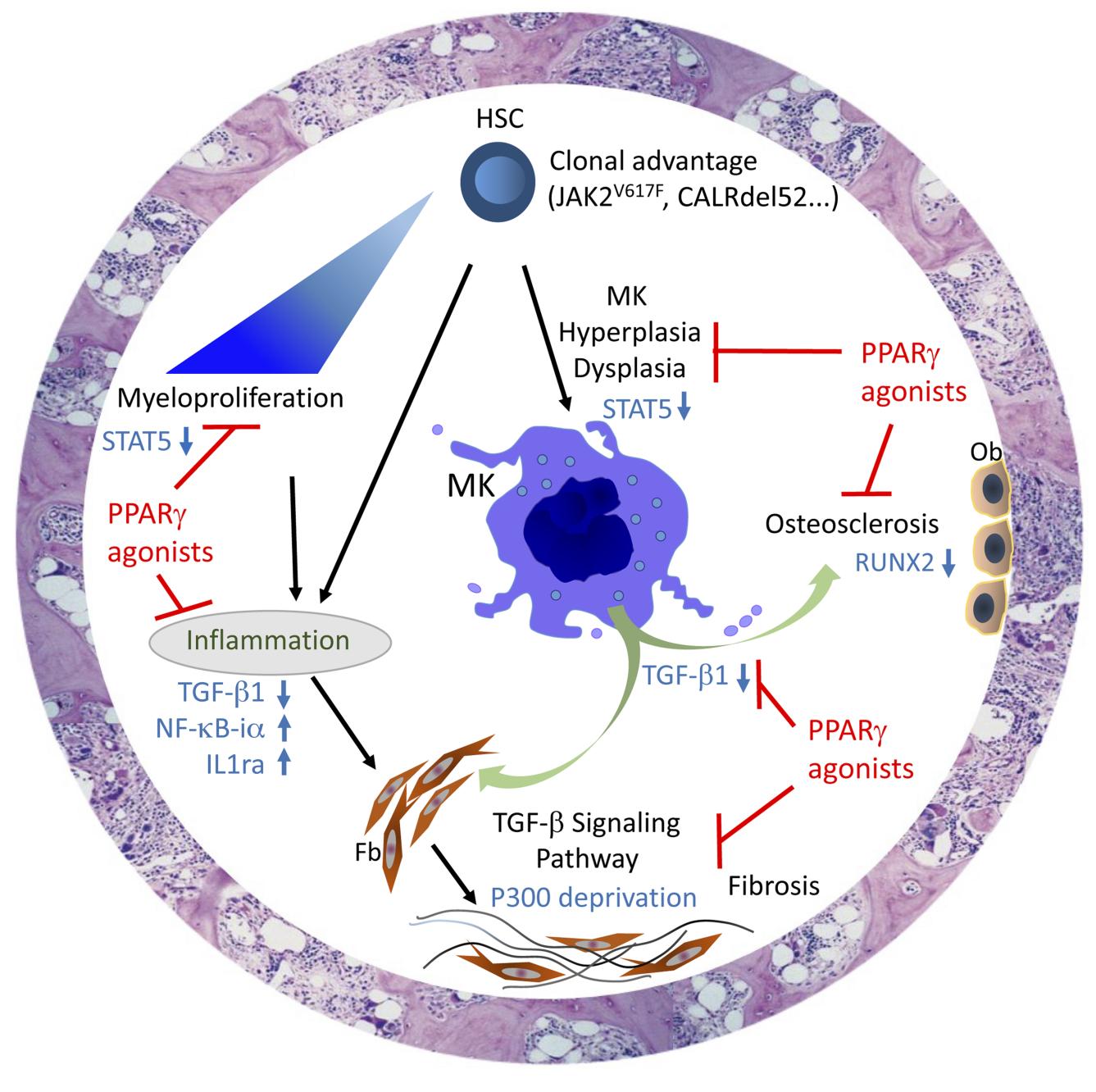
By acting on these three key axes of myelofibrosis development, PPAR-γ proves itself as a pertinent therapeutic target in disease management. The results published by the LCSAT team lend credence to the use of PPAR-γ agonists in clinical practice.
This work was carried out by a CEA-Jacob team that is part of the OPALE consortium, a Carnot Institute dedicated to R&D in the field of leukemia and related diseases.
1 : The formation of all of the blood cells within the various blood cell lines begins in the bone marrow. The hematopoietic stem cells give rise to two progenitor lines, the myeloid lineage and the lymphoid lineage. The myeloid lineage will ultimately differentiate into platelets, which participate in coagulation, red blood cells, which carry oxygen to tissues, and two types of white blood cells, granulocytes and monocytes, which play roles in immunity and the fight against infections.
2 : Myelofibrosis is described as secondary when it develops on a background of another pathology such as essential thrombocythemia or polycythemia vera.
3 : Institut de biologie François Jacob - Une thérapie pour vaincre définitivement la leucémie myéloïde chronique (LMC) (cea.fr)
4 : In partnership with the Hematology and Oncology Department of the Versailles Hospital Center, the Clinical Investigations Center of Saint Louis Hospital (AP-HP), the Institute of Molecular Genetics of Montpellier, the Gustave Roussy Institute and the Pharmacology-Toxicology Department of Raymond Poincaré Hospital.
5 : One of the first signs of myelofibrosis is anemia, which may be diagnosed via a reduction in the amount of hemoglobin in the blood.
6 : Myeloproliferation describes the clonal proliferation of hematopoietic progenitor cells and myeloid lineage blood cells.
7 : Stroma describes the sponge-like tissue in the medullar cavity of bone wherein hematopoietic stem cells multiply and differentiate. It is composed of stromal cells (fibroblasts, adipocytes, osteoblasts), a microvasculature (endothelial cells) and derived structures and molecules (extracellular matrix, hematopoietic growth factors).