Metallic nanoparticles (gold, platinum, hafnium, and gadolinium) are increasingly studied as radiosensitizers able to enhance the effects of radiotherapy. However, the cellular mechanisms governing their internalization remain poorly understood.
In this study, researchers from the Genetic Stability, Stem Cells and Radiation Unit from IRCM compared the uptake of nanoparticles (gold and TiO₂) in several breast cancer cell lines with distinct phenotypes. Their results show that:
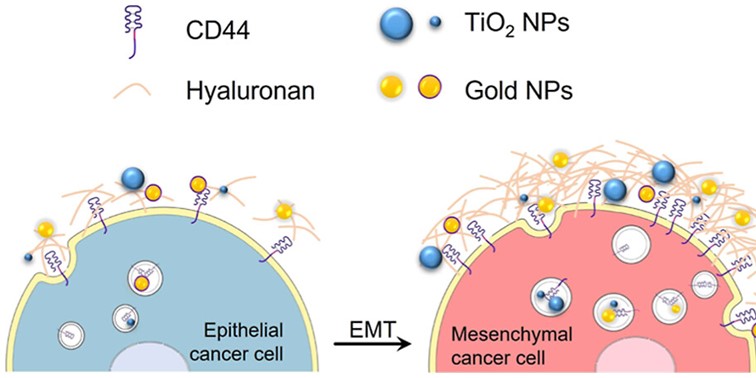
- Mesenchymal cells consistently internalize more nanoparticles than epithelial cells, regardless of nanoparticle type or size;
- In vitro models confirm that the transition to a mesenchymal state significantly increases nanoparticle endocytosis;
- Transcriptomic analysis identify more than 500 differentially expressed genes, with a mesenchymal signature strongly linked to extracellular matrix organization;
- Among these genes, HAS2 (hyaluronan synthase 2) is highly overexpressed in mesenchymal cells, boosting hyaluronan (HA) production;
- The CD44s receptor, abundant in mesenchymal cells, directly contributes to nanoparticle uptake: its inhibition or HA degradation drastically reduce uptake;
@ E. Bourneuf / CEA
These observations extend to a broader panel of tumor cell lines, confirming that the HA–CD44 axis constitutes a preferential entry route for nanoparticles in mesenchymal-like breast cancer cells.These findings highlight a key cellular mechanism that could be harnessed to specifically target the most aggressive and treatment-resistant tumor cells, paving the way for more selective and effective radiotherapy strategies.
Contact : emmanuelle.bourneuf@cea.fr