Genomic stability depends on the ability of cells to repair DNA double-strand breaks while protecting the natural ends of chromosomes—the telomeres—from the same repair machinery. In the yeast Saccharomyces cerevisiae, as in mammals, this protection prevents chromosomal fusions that would compromise cell survival.
The Ku70/Ku80 complex (known as Ku) plays a paradoxical role: it is essential for repairing double-strand breaks through non-homologous end joining (NHEJ), yet it also binds constitutively to telomeres without triggering fusions. The mechanism allowing this coexistence had remained unclear.
The team led by Stéphane Marcand at the DRCM of the Institut Jacob has shown that the Rap1 protein, the main DNA-binding factor at telomeres, acts as a "lock" that restricts Ku movement along the DNA:
- a single Rap1 binding site placed near a break is enough to block NHEJ repair activity without preventing Ku from binding;
- in vitro experiments (EMSA, cryo-EM, molecular modeling) show that Rap1 and Ku can coexist on the same DNA fragment, but that Rap1 prevents Ku from “sliding” inward along the double helix—an essential step for end ligation during repair;
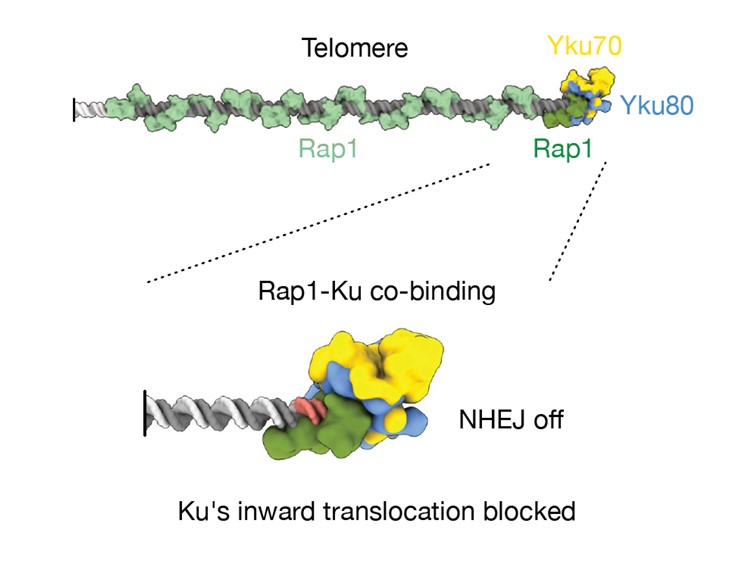
- cryo-EM imaging reveals a Rap1–DNA–Ku complex in which Rap1 acts as a physical barrier, limiting Ku translocation by about 25 base pairs;
- nanopore sequencing of telomeric fusions confirmed in vivo that telomeres where Rap1 is positioned either too close or too far from the chromosome end are significantly more prone to fusions, highlighting the importance of its precise positioning.
This mechanism converts Ku from a potentially hazardous repair factor into a protective guardian of telomeres. By restricting Ku movement, Rap1 prevents accidental end-joining between chromosomes while preserving the protective function of the Ku complex.
@ S. Marcand / CEA
Proposed model for telomere protection by the Rap1 protein located near the chromosome end.
At telomeres, the restriction of Ku’s inward translocation by proximal Rap1 protects chromosome ends. Yku70 is shown in yellow, Yku80 in blue, Rap1 bound to the proximal telomeric site in green, other Rap1 molecules bound along the telomere in light green, telomeric DNA in grey, and the Rap1 binding site highlighted in salmon (on both DNA strands).
This study reveals a universal principle of telomere protection based on the spatial modulation of DNA–protein interactions. It sheds light on how organisms maintain long-term chromosomal stability and suggests that similar mechanisms may operate in mammals.
Contact: Stéphane Marcand