Human hematopoiesis—the production of blood cells from stem cells—takes place in the bone marrow, a complex microenvironment that is difficult to reproduce experimentally. Traditional murine models for studying human hematopoiesis use immunodeficient mice. While they allow progress, their physiological relevance is limited since human cells develop within mouse bone marrow.
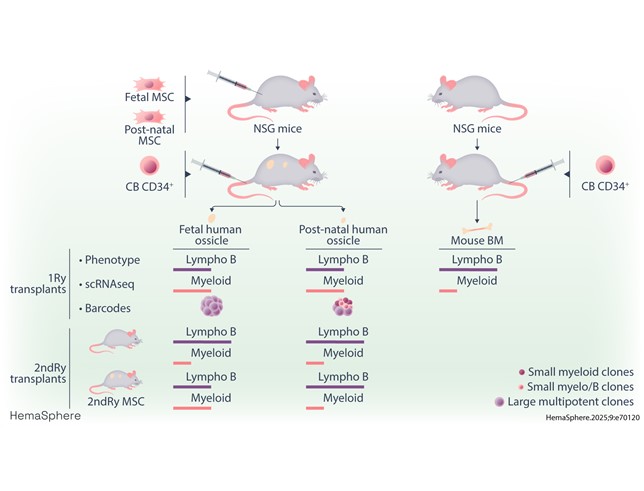
In this study, the DRCM team developed and characterized an alternative model: humanized ossicles (hOss), bone-like structures derived from human mesenchymal stem/stromal cells isolated before or after birth and implanted into immunodeficient mice. After injection of CD34+ hematopoietic stem/progenitor cells, these hOss effectively support multi-lineage human hematopoiesis.
The results show that:
hOss promote the production of myeloid cells (granulocytes, monocytes) and erythrocytes over B lymphocytes, more faithfully reflecting the proportions observed in human bone marrow compared to conventional murine models.
hOss contain re-isolatable mesenchymal stem cells capable of forming secondary hOss with functional niches for human hematopoiesis.
Stem cells recovered from hOss display an enhanced blood regeneration potential compared to those isolated from murine bone marrow.
Clonotypic tracking using genetic barcodes reveals exchanges and complementarity between hOss and mouse marrow, but with a specific contribution of hOss to supporting myeloid and multipotent lineages.
Differences emerge depending on whether stromal cells are of fetal or postnatal origin: fetal hOss appear to provide a more balanced and durable environment for hematopoietic production.
These results establish hOss as a robust and physiologically relevant model for studying the normal and pathological development of human blood cells, as well as metastatic mechanisms of solid tumors. The study therefore opens up new perspectives for research on leukemia, on protective interactions between cancer cells and bone marrow cells, and may help to pave the way for personalized therapies.
Contact : Françoise Pflumio ; Laurent Renou