Maintaining continuous surveillance, which involves the coordinated activation of innate and adaptive immune responses, is a cornerstone of the immune system's fundamental role of protecting the organism. Normally, a developing cancer (neoplastic transformation) will incite the immune system to activate and attack the tumor. However, this latter, according to its mutation profile, will be more or less susceptible to that attack.
Immunotherapies, notably immune checkpoint inhibitors1, are currently privileged treatments for making anti-tumor immune responses as efficacious as possible in certain types of cancers.
The inhibitory effects of these treatments are provided by therapeutic immunoglobulins, the best-known of which are the anti-PD1/PDL11 antibodies. However, despite globally encouraging results, some patients do not respond to these immunotherapies. This may be due to the presence, within the tumor, of multiple mechanisms involving different immune checkpoints, that, together, enable immune response evasion and make therapies targeting only one of those mechanisms inefficacious.
For several years now, researchers from the Immuno-Hematology Research Unit (SRHI) have been studying the molecule HLA-G, which is involved in immune system tolerance, particularly maternal-fetal tolerance. Indeed, under normal conditions, this molecule is only expressed during pregnancy on the surface of placental cells. It suppresses the immune system by interacting with the ILT2/ILT4 receptors present on the surface of immune cells. However, HLA-G expression is also detected in several other settings, including certain neoplasms, where it affords cancer cells protection from the host's immune system and resultantly the opportunity to develop or relapse. HLA-G expression by cancer cells may also explain cases of resistance to current immunotherapies. Indeed, in an earlier study, the SRHI team had shown that the use of anti-HLA-G antibodies restored tumoral immune response in a preclinical murine model.
The objective of the SRHI research in this context is to determine the interest of the HLA-G/ILT2/ILT4 immune checkpoint over others when patients are unresponsive to anti-PD1/-PDL1 therapies, and consequently the utility of anti-HLA-G antibodies as an efficacious complementary immunotherapy.
In their most recent article published in Cancer Letters, the SRHI researchers sought a better understanding of the effects of tumor HLA-G expression on tumor-infiltrating T lymphocytes2 in the setting of clear cell renal cell carcinoma (ccRCC).
They showed that CD8+ and CD4+ TILs taken from patients with ccRCC also expressed the ILT2 receptor (ILT2+) with which HLA-G interacts.
Interestingly, Patients with CD4+/ILT2+ T lymphocytes within their tumors had less of them elsewhere, suggesting the preferential accumulation of those cells in the neoplastic tissue. Furthermore, CD4+/ILT2+ TILs expressed cytotoxic markers such as perforin, KLRG1, GPR56 and Tbet, which are normally found on CD8+ cytotoxic T lymphocytes and NK (natural killer) cells involved in apoptosis.
The CD4+/ILT2+ TILs identified in situ also showed marker specificities (CD27- CD28- CCR7- CD45RA+) and delayed differentiation suggesting a phenotype characteristic of that of effector memory T cells, a subset of memory T cells able to deploy a more rapid and efficacious immune response.
The researchers also reported for the first time that CD4+/ILT2+ TILs are indeed functionally cytotoxic, but they lose that quality when exposed to HLA-G-presenting cells. That finding suggests that the cytolytic activity of CD4+/ILT2+ TILs may be restored by blocking the HLA-G/IL2 interaction.
The clinical potential of those cells was confirmed via immunohistochemical analyses on tumor lesion samples taken from patients with ccRCC. With those analyses, the team observed HLA-G+ tumor cells in the zones infiltrated by the CD4+ T cells.
These recent results published by the SRHI team show that CD4+/ILT2+ TILs represent a reservoir of cytotoxic T cells within the tumor that are not addressed by current checkpoint inhibitors. That could change however with the use of therapeutic antibodies designed to hamper HLA-G/ILT2 interactions. Their study thus lays a pathway to novel alternative therapies for HLA-G+ tumors.
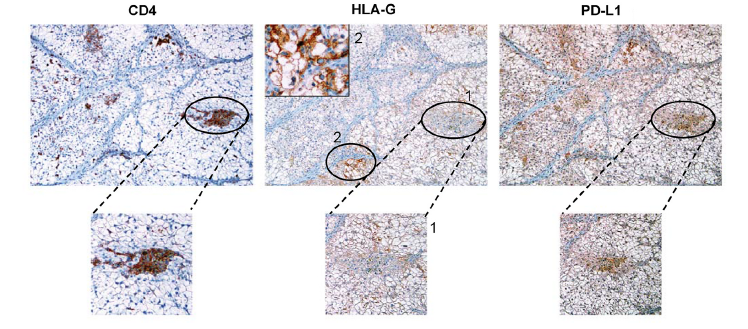
Immunohistochemical analysis of co-expression of CD4, HLA-G and PDL1 markers on serial sections of tumor tissue from a patient with clear cell renal cell carcinoma (SRHI)
1: The term immune checkpoint describes a number of molecular mechanisms to prevent immune cells, such as T lymphocytes, from being continuously activated and destroying healthy cells, as occurs in autoimmune diseases. These checkpoints can be thought of as locks that, once closed by their respective keys, prevent immune cells from being activated. In some cases, tumor cells may get their metaphorical hands on one of those keys and use it to close the lock, thus protecting themselves from attack by the immune system. PDL1 is one of the best-known of these keys expressed by tumor cells. These latter use it to "lock" PD1, an immune checkpoint residing on the surface of immune cells. To prevent tumors from doing this, researchers have developed immune checkpoint inhibitors, that is, anti-PD1 and anti-PDL1 antibodies that prevent the key from going in the lock. In so doing, the immune cells remain able to identify the cancer cells as such and mount an immune response against them.
2: T lymphocytes (TLs) play roles in adaptive immune responses; they are major actors in cell-mediated immunity. There are several types of TLs, including cytotoxic CD8+ TLs that destroy virus-infected or cancer cells and "helper" CD4+ TLs that act as immune response intermediaries to guide other lymphocytic functions.
This study was financed by the French National Cancer Institute and the result of a close partnership between the researchers at SRHI (CEA-Jacob) and the oncologists, urologists and anatomical pathologists at the Saint-Louis Hospital in Paris.