The vast majority of human cancers, 90% of them, involve solid tumors. In some of them, increased expression of cellular prion protein (PrPC) is associated with poor prognosis. Indeed, at the cellular level, high PrPC expression rates are correlated with the acquisition of stemness¹, invasive & metastatic potential and chemotherapy resistance. However, the role of PrPC in tumoral response to radiation therapy has not yet been clearly described.
In a study published in Oncogene, researchers from the Research Laboratory on Repair and Transcription in Hematopoietic Stem Cells (LRTS) and the Laboratory of Genetic Instability Research (LRIG), both within IRCM's Genetic Stability, Stem Cells and Radiation (SGSCR) Mixed Research Unit, showed that in a number of solid tumors (breast, colorectal neuroblastoma²), acute or fractionated exposure to ionizing radiation (IR) increases expression of PRNP, the gene that codes for PrPC. They also showed that when that expression is limited (via RNA interference) or abolished (via CRISPR-Cas9 gene editing), the tumors become more radiosensitive, that is, more likely to respond to radiation therapy.
Exposing a cell to IR causes breaks in its DNA. This evokes the cell's DNA damage response (DDR), which is activated mainly by the recruitment and activation of the ataxia-telangiectasia mutated (ATM) protein kinase. This latter, in turn, via a phosphorylation cascade, can activate hundreds of downstream targets to bring about cell cycle arrest and/or apoptosis and/or DNA repair according to the initial damage and the cell context.
With that in mind, the researchers sought to characterize the molecular mechanisms behind the IR-driven PRNP upregulation in neuroblastoma cells, focusing their attention particularly on the DDR.
They thus showed that in those cells, IR activates a signaling pathway involving ATM protein kinase, transforming growth factor β-activated kinase 1 (TAK1) and c-Jun N-terminal kinase 1(JNK1). That signaling pathway activates PRNP transcription via the binding of the AP-1 transcription factor at its site on the gene's promoter³ region.
Furthermore, this radioresistance-associated ATM/TAK1/JNK1 signaling pathway was found to be activated after irradiation in the other types of cancer cells that the team studied. Finally, the researchers demonstrated increased sensitivity to irradiation in breast, colorectal and neuroblastoma cancer cells wherein upregulation of PRNP expression was prevented by the pharmacological inhibition of the TAK1 protein.
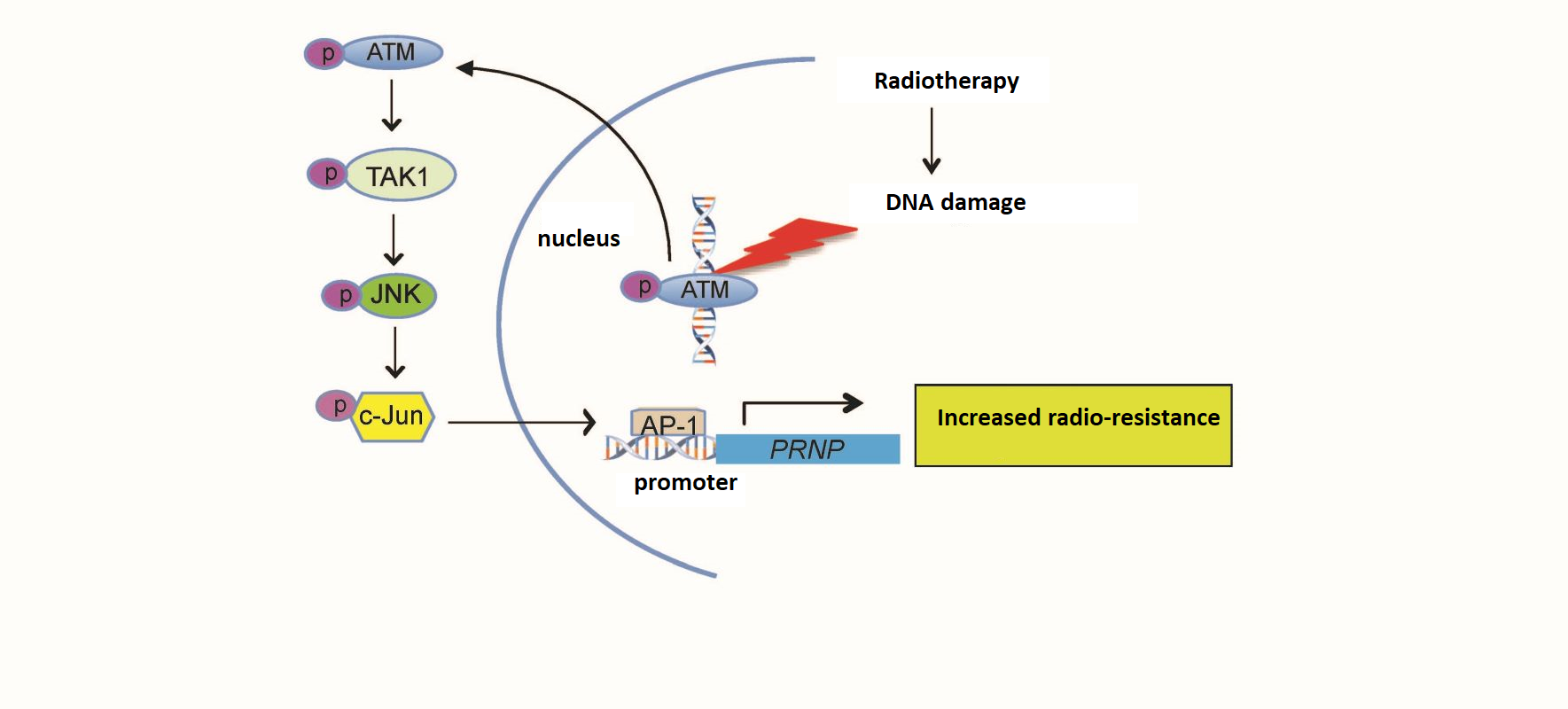
With their demonstration of the role of the ATM/TAK1/JNK1 signaling pathway in the increased expression of cellular prion protein, the LRTS-LRIG team has shed new light on the molecular mechanisms driving radioresistance in solid tumor cancers.
1 : Said of cells showing some level of pluripotency, that is, an ability to differentiate into a range of cell types.
2 : Neuroblastoma is a neural but extra-cerebral malignant solid tumor. It is the most common cancer in infants. Neuroblastomas are the cancerous degeneration of neuroblasts, which give rise to the sympathetic nervous system. They develop frequently around the spinal column.
3 : A promoter is a region of DNA situated close to and essential for a gene. Promotor regions are a sort of molecular launch point for the transcription of DNA into RNA.